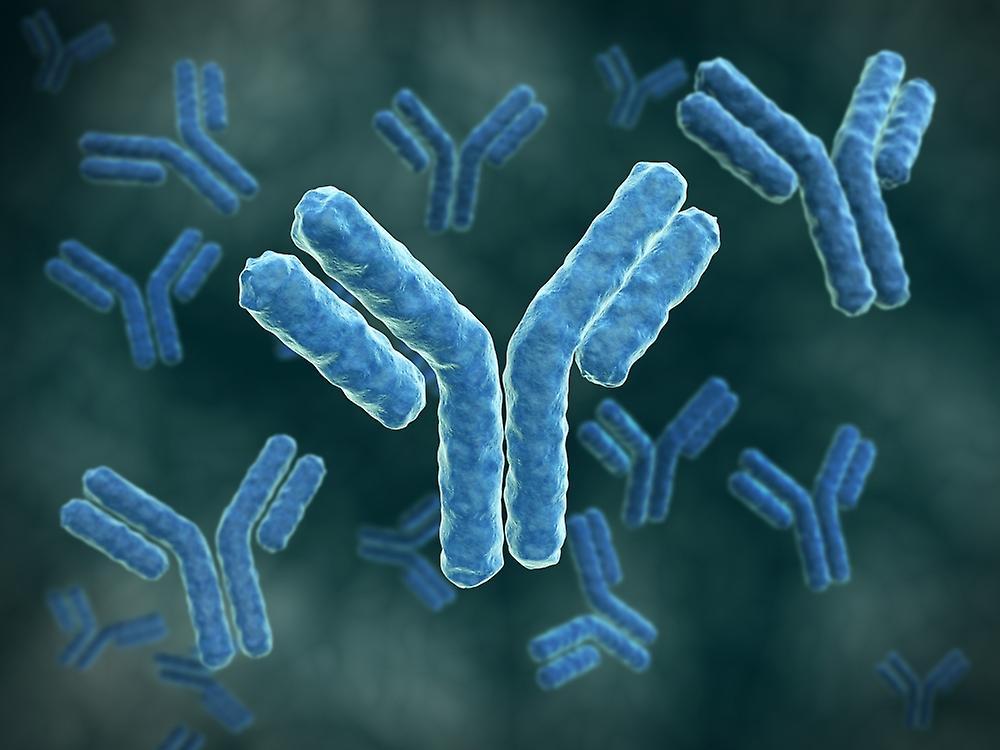
Structure and Function
Immunoglobulin, commonly known as antibodies, are Y-shaped proteins produced by plasma cells that are involved in the defense against foreign pathogens like viruses and bacteria. The basic structure of an antibody molecule consists of two light chains and two heavy chains that are linked together by disulfide bonds. The types of immunoglobulin light chains can be of two - kappa or lambda, while the heavy chains can be divided into five main classes - IgA, IgD, IgG, IgE, and IgM. Each arm ends in an antigen-binding fragment (Fab) that specifically binds to antigens like proteins or polysaccharides. The tail region called the Fc fragment interacts with other parts of the immune system to mediate effector functions.
Role of Different Immunoglobulin Classes
IgG is the most abundant class found in blood and extracellular fluid, constituting about 75% of serum Immunoglobulin. With its ability to cross the placenta, IgG provides protection to babies through maternal antibodies. IgG also destroys pathogens through complement activation and phagocytosis.
IgM is present as a pentamer in blood and is the first antibody produced when primary infection occurs. As it activates complement easily, IgM plays an important role in clearing bacterial infections.
IgA exists as a dimer and predominates in bodily secretions like saliva, tears, breast milk etc. It provides mucosal immunity against pathogens trying to enter through these surfaces.
Get more insights on- Immunoglobulin
Explore More Related Article On- Immunotherapy Drugs Market